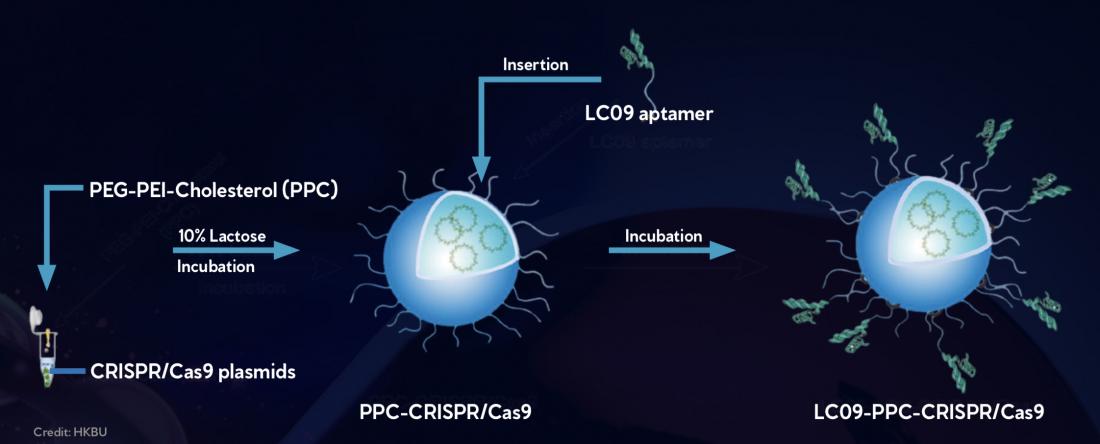
CRISPR/Cas9 gene editing compounds are mixed with a lipid polymer solution (PPC) (left), which leads to CRISPR/Cas 9 being spontaneously encapsulated by a protective lipid layer (center). Then molecules designed to specifically recognize osteosarcoma cells, called LC09 aptamers, are added to the exterior of the lipid layer (right). This enables the CRISPR/Cas9 compounds to specifically target and edit a gene controlling blood vessel formation in osteosarcoma cells, thereby limiting tumour growth.
Researchers at Hong Kong Baptist University (HKBU) (HKBU) and colleagues have developed a delivery system to transport a gene editing technology to osteosarcoma cells and their metastases, depriving them of their ability to form new blood vessels.
The system is comprised of peptides that specifically target osteosarcoma cells, and a protective fatty coating for CRISPR/Cas9 gene editing compounds, helping them evade degradation in the body. Designed by Ge Zhang of HKBU’s Institute for Advancing Translational Medicine in Bone and Joint Diseases and his team, the system has been tested in cultured mouse and human osteosarcoma cells, and in mice with osteosarcoma and its lung metastases.
Osteosarcoma is a highly aggressive bone cancer in children and adolescents. It is often complicated by bone destruction and fracture, while cells detaching from the tumour can also settle in other tissues—a process called metastasis. Osteosarcoma metastasis significantly reduces chances of living beyond five years from first diagnosis.
Vascular endothelial growth factor A (VEGFA) is a gene that is highly expressed in osteosarcomas. It encodes a protein that facilitates new blood vessel formation and tumour cell survival. Targeting VEGFA with the CRISPR/Cas9 gene editor – which can edit DNA by snipping out specific nucleotide sequences – is expected to suppress osteosarcoma growth and metastasis, but it has been challenging to develop targeted delivery systems.
To overcome this, the researchers encapsulated a VEGFA gene editor in a protective lipopolymer coating composed of polyethylenimine, methoxypolyethyleneglycol, and cholesterol. The coating, called PPC, protects the gene editor from degradation by the body’s enzymes as it travels to its destination. Peptide molecules designed to specifically recognise and target osteosarcoma cells were attached to the lipopolymer coating.
The delivery system successfully targeted osteosarcoma tissues and their lung metastases without accumulating in other tissues. The CRISPR/Cas9 gene editing technology decreased VEGFA expression and secretion, reduced new blood vessel formation in osteosarcoma tumour tissue, and inhibited tumour growth and metastasis.
The researchers conclude in their study published in the journal Biomaterials that their work may pave the way for new clinical approaches using the CRISPR/Cas9 gene editing technology in cancer treatment. They next plan to test the system in a mouse model of human cancer and then move to clinical trials if the results are promising.
For further information, contact:
Professor Ge Zhang
Institute for Advancing Translational Medicine in Bone and Joint Diseases
Hong Kong Baptist University (HKBU)
E-mail: [email protected]